Answer:
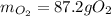
Step-by-step explanation:
Hello.
In this case, given the chemical reaction, we can compute the grams of oxygen by using the 98.2 g of water via the 2:1 mole ratio between them, the molar mass of water that is 18.02 g/mol, the molar mass of gaseous oxygen that is 32.00 g/mol and the following stoichiometric procedure relating the given information:
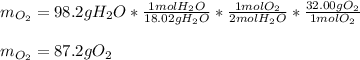
In which the result is displayed with three significant figures because the given mass of water 98.2 g, has three significant figures too.
Best regards!