Answer:
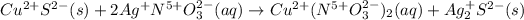
It is a non-redox reaction.
Step-by-step explanation:
Hello.
In this case, when solid copper sulfate and aqueous silver nitrate react to form solid silver sulfide and aqueous solid silver sulfide we can write:
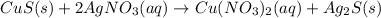
In which the oxidation states are assigned based on the periodic table and taking into account that the left-handed ion is positively charged whereas the right-handed one is negatively charged:
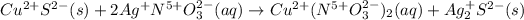
Thus, since the oxidation states do not change from reactants to products, we infer this is a non-redox reaction.
Best regards!