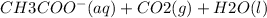Answer:
The active ingredients in baking soda (NaHCO3) are
 and
and
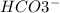
when Baking soda reacts with Acetic acid
Molecular equation
NaHCO3(aq) + CH3COOH(aq) → Na(CH3COO)(aq) + CO2(g) +H2O(l)
Ionic equation
 →
→
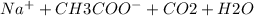
as
 is present on both sides so it will cancel out and the net ionic equation will be
is present on both sides so it will cancel out and the net ionic equation will be
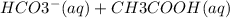 →
→