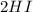Answer:
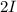 will cancel out and the overall reaction will be
will cancel out and the overall reaction will be
 →
→
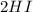
Step-by-step explanation:
The gas phase reaction between hydrogen and iodine is proposed to occur as follows
Step 1 fast
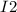 ⇄
⇄
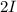
step 2 slow
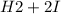 ⇄
⇄
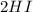
The overall reaction for these step reaction will be
 →
→