Answer:
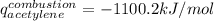
Step-by-step explanation:
Hello.
In this case, for this calorimetry problem, since the acetylene is burned into the calorimeter causing a temperature increase, we can infer that the combustion of acetylene releases heat which is absorbed by the calorimeter, therefore, the following equation can be written:
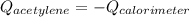
Which can be written in terms of the energy of combustion and moles of the acetylene and the heat capacity and temperature change of the calorimeter:
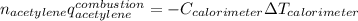
Thus, since the molar mass of acetylene is 26.04 g/mol, the resulting energy of combustion of the acetylene turns out:
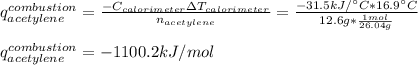
Best regards!