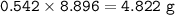Mass of Al₂(SO₄)₃ : 4.822 g
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
2AlCl₃ + 3(NH₄)₂SO₄⇒Al₂(SO₄)₃+ 6NH₄Cl
MW AlCl₃ :133,34 g/mol
MW (NH₄)₂SO₄ : 132,14 g/mol
MW Al₂(SO₄)₃ : 342,15 g/mol
mol AlCl₃
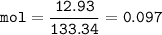
mol (NH₄)₂SO₄
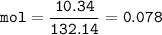
Limitng reactants (ratio mol : coefficient = the smaller)
AlCl₃ : (NH₄)₂SO₄ =
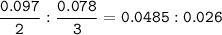
(NH₄)₂SO₄ ⇒ limiting reactants
So mol Al₂(SO₄)₃ from (NH₄)₂SO₄
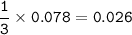
mass Al₂(SO₄)₃
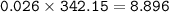
with 54.2% yield, the mass of Al₂(SO₄)₃