Answer:
Missing particles:
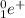 (a positron) and an electron neutrino
(a positron) and an electron neutrino
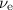 .
.
The nuclear equation would be:
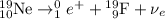 .
.
Step-by-step explanation:
The mass number of a particle is the number on the top-right corner of its symbol.
The atomic number of a particle is the number on the lower-right corner of its symbol.
The nuclear reaction here resembles a beta-plus decay. The mass numbers of the two nuclei are equal. However, the atomic number of the product nucleus is lower than that of the reactant nucleus by
 .
.
A beta decay may either be a beta-plus decay or a beta-minus decay. In a beta-plus decay, a positively-charged positron
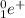 and an electron neutrino
and an electron neutrino
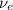 would be released. On the other hand, in a beta-minus decay, a negatively-charged electron
would be released. On the other hand, in a beta-minus decay, a negatively-charged electron
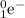 and an electron antineutrino
and an electron antineutrino
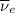 would be released.
would be released.
Electric charge needs to be conserved in nuclear reactions, including this one.
The atomic number of the
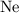 nucleus on the left-hand side is
nucleus on the left-hand side is
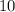 , meaning that the nucleus has a charge of
, meaning that the nucleus has a charge of
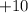 . On the other hand, the atomic number of the
. On the other hand, the atomic number of the
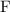 nucleus on the right-hand side shows that this nucleus carries a charge of only
nucleus on the right-hand side shows that this nucleus carries a charge of only
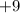 .
.
By the conservation of electric charge, the particles on the right-hand side must carry a positive charge of
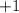 . That rules out the possibility of the combination of one negatively-charged electron
. That rules out the possibility of the combination of one negatively-charged electron
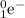 (with a charge of
(with a charge of
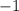 ) and an electron antineutrino
) and an electron antineutrino
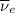 (with no electric charge at all.)
(with no electric charge at all.)
Hence, the only possibility is that the missing particle is a positron (and an electron neutrino
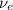 , which carries no electric charge.)
, which carries no electric charge.)