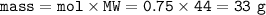Mass of CO₂ = 33 g
Further explanation
Complete combustion of Hydrocarbons with Oxygen will be obtained by CO₂ and H₂O compounds.
If O₂ is insufficient there will be incomplete combustion produced by CO and H and O
Reaction
CH₄ + 2O₂⇒CO₂ + 2H₂O
mol CH₄ :
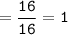
mol O₂ :
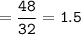
A method that can be used to find limiting reactants is to divide the number of moles of known substances by their respective coefficients
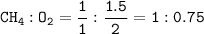
Because O₂ ratio smaller then O₂ becomes the limiting reactants
So mol CO₂ from limiting reactants
mol CO₂ :
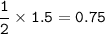
mass CO₂ :