Answer:
a)
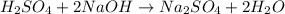
b)
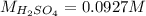
Step-by-step explanation:
Hello!
In this case, for this acid-base reaction which also known as a neutralization because sulfuric acid is neutralized with sodium hydroxide, we can write the undergoing chemical reaction as shown below:
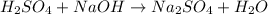
However, it needs to be balanced as two sodium atoms are yielded:
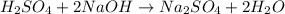
Next, since there is a 1:2 mole ratio between the acid and the base, at the equivalence point, at which the moles of acid and base are consumed, we write:
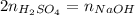
Which can also be written in terms of the given volumes and concentration of the base:
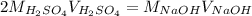
In such a way, we solve for the concentration of sulfuric acid as shown below:
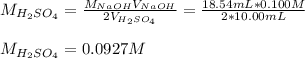
Best regards!