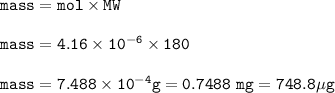Grams of glucose = 0.7488 mg=748.8 μg
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
6CO₂+6H₂O⇒C₆H₁₂O₆+6O₂
25 micromoles, μmoles, of CO₂ :
moles CO₂ : moles glucose - C₆H₁₂O₆ = 6 : 1, so :
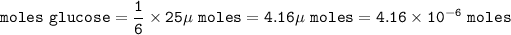
mass of glucose