Answer:
55.4g
Step-by-step explanation:
Given parameters:
Number of atoms in the bottle = 8.34 x 10²²atoms
Unknown:
Mass of air in the bottle = ?
Solution:
The given specie is argon.
Molecular mass of Argon = 40g/mol
To find the mass of this Argon ;
Mass = number of moles x molecular mass;
Let us find the number of moles of the Argon;
6.02 x 10²³ atoms can be found in 1 mole of any substance
8.34 x 10²² atoms will give
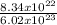 mole of Argon
mole of Argon
= 0.139mole of Argon
So;
Mass of argon = 40g/mol x 0.139mole = 55.4g