Answer:
3 moles
Step-by-step explanation:
The balanced chemical equation between Calcium Hydroxide and Hydrochloric acid is:
Ca(OH)₂ + 2HCl → CaCl₂ + 2H₂O
The ratio of HCl to H₂O is 2 : 2
So if 3 moles of hydrochloric acid is added, 3 moles of water will be formed:
[x is the no of moles of H₂O produced]
2 : 2
3 : x
[Cross multiply]
2x = 2 × 3
2x = 6
x =
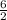 = 3 moles
= 3 moles