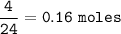0.16 moles
Further explanation
There are 2 conditions that are usually used as a reference in chemical calculations (mainly for determining the volume per mole of gas or the molar volume), namely:
stated by STP (Standard Temperature and Pressure). At STP, Vm is 22.4 liters/mol.
stated by RTP (Room Temperature and Pressure). Vm in this condition = 24.4 liters / mol
so for 4 L :