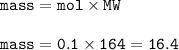The mass of solute : 16.4
Further explanation
Molarity is a way to express the concentration of the solution
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
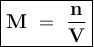
Where
M = Molarity
n = number of moles of solute
V = Volume of solution
mol Na₃PO₄
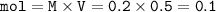
mass Na₃PO₄