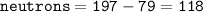Neutrons = 118
Further explanation
The elements in nature have several types of isotopes
Isotopes are elements that have the same Atomic Number (Proton)
Atomic mass is the average atomic mass of all its isotopes
The most abundant isotope of gold (Au) is isotope Au-197
So the atomic mass of Au = 197
and the atomic number of Au = 79
Atomic mass is the sum of protons and neutrons
Atomic Number (Z) = Atomic mass (A) - Neutrons
Neutrons = A - Z
So neutrons for Au-197 :