Answer:
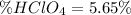
Step-by-step explanation:
Hello,
In this case, since the reaction between barium hydroxide and perchloric acid is:
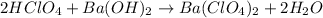
It means there is a 2:1 mole ratio between the acid and the base; thus we compute the moles of barium hydroxide that are reacting:
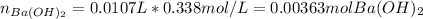
Now, we compute the mass of perchloric acid (molar mass = 100.46 g/mol) by considering that 2:1 mole ratio:
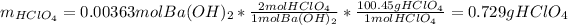
Finally, the percent of perchloric acid in such sample is:
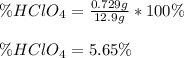
Best regards!