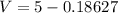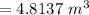Answer:
The correct answer is "4.8137 m³". The further explanation is given below.
Step-by-step explanation:
Firstly we have to calculate the concentration of Se:
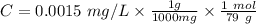
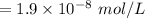
Concentration the fish can take:
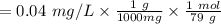
According to the general dilution principle will be:
⇒
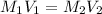
The volume that can take the farmer will be:
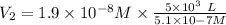
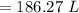
On converting this into m³, we get
=
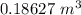
Finally the volume the farmer can remove would be: