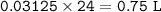Concentration Ca(OH)₂ : 1.25 M
Volume of H₂ : 0.75 L
Further explanation
The reaction equation is the chemical formula of reagents and product substances
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products
Reaction
Ca(s) + 2H₂O(1) ⇒ Ca(OH)₂(aq) + H₂(g)
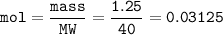
- concentration of Ca(OH)₂ :
mol Ca = mol Ca(OH)₂ = 0.03125
V = 25 cm³ = 0.025 dm³(L)
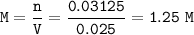
Assume At RTP (room temperature and pressure), 1 mol gas = 24 L
mol H₂ = mol Ca = 0.03125
volume :