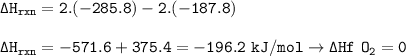The enthalpy change : -196.2 kJ/mol
Further explanation
The change in enthalpy in the formation of 1 mole of the elements is called enthalpy of formation
The enthalpy of formation measured in standard conditions (25 ° C, 1 atm) is called the standard enthalpy of formation (ΔHf °)
(ΔH) can be positive (endothermic = requires heat) or negative (exothermic = releasing heat)
The value of ° H ° can be calculated from the change in enthalpy of standard formation:
∆H ° rxn = ∑n ∆Hf ° (product) - ∑n ∆Hf ° (reactants)
Reaction
2 H₂O₂(l)-→ 2 H₂O(l) + O₂(g)
∆H ° rxn = 2. ∆Hf ° H₂O - 2. ∆Hf °H₂O₂