Answer:
17.5 moles of oxygen gas
Step-by-step explanation:
Propane burns with the help of oxygen according to the following reaction
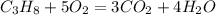
It can be seen that for 1 mole of propane 5 moles of oxygen is required.
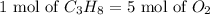
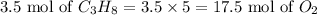
The reaction will be
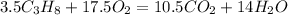
Therefore 17.5 moles of oxygen gas will be needed to burn 3.5 moles of propane.