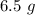Answer:
Mass of NO produced is "6.5 g".
Step-by-step explanation:
The given reaction is:
⇒
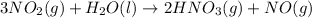
Now,
⇒
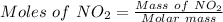
⇒
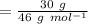
⇒
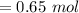
- We shouldn't have to acknowledge the sum of H₂O as it would be in excess. It's going to mot finish resolving answer.
- We provided 0.65 mol of NO₂, of which 3 mol of NO₂ = 1 mol of NO was previously given.
Accordingly,
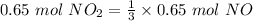 is created.
is created.
So,
The mass of NO will be:
=
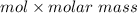
=
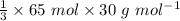
=