Answer:
The energy of the photon is 1.718×10−26J.
Step-by-step explanation:
The energy of the photon is
1.718 × 10 − 26J. You use the formula E =
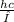 , where h is Planck's Constant,c is the speed of light, and λ is the wavelength of the photon.
, where h is Planck's Constant,c is the speed of light, and λ is the wavelength of the photon.
E=
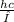 =
=
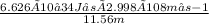 = 1.718×10−26J.
= 1.718×10−26J.