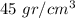Answer:
The volume of the block is
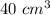
The density of the block is
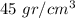
Step-by-step explanation:
Density
Is a measure of the mass m of a body per unit volume V it occupies.
Its formula is
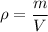
Metal A has a density of
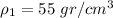 and metal B has a density of
and metal B has a density of
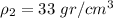 .
.
We know m1=1200 gr of metal A and m2=600 gr of metal B are melted, mixed, and recast into a block.
Assuming no mass loss occurs and both metals are uncompressible, the total mass is the sum of the individual masses, and the total volume is the sum of both volumes.
The total mass can quickly be calculated:
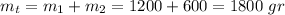
a)
The volumes will be calculated by using the provided formula, and solving it for V:
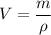
For metal A:
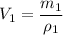
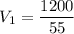
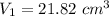
Now for Metal B:
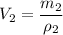
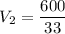
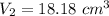
The total volume is:
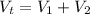
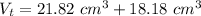
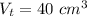
The volume of the block is
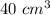
b) The density of the block is the total mass by the total volume:
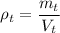
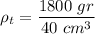
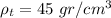
The density of the block is