Answer:

Step-by-step explanation:
We are given the chemical formula:
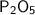
The subscript of 2 comes after the P. The element P is phosphorous, so there are 2 phosphorous atoms.
The subscript of 5 comes after the O. The element O is oxygen, so there are also 5 oxygen atoms.
Therefore the correct answer is A. 2 phosphorous and 5 oxygen