Answer:
4.5g
Step-by-step explanation:
Given parameters:
Mass of copper(i)chloride = 6.93g
Unknown:
Mass of copper = ?
Solution:
Formula of the compound = CuCl
atomic mass of Cu = 63.6g/mol, atomic mass of Cl = 35.5g/mol
Molecular mass = 63.6 + 35.5 = 99.1g/mol
So,
The mass of copper =
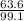 x 6.93 = 4.5g
x 6.93 = 4.5g