Answer:
315 mL
General Formulas and Concepts:
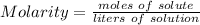
Step-by-step explanation:
Step 1: Define variables
0.555 M NaHCO₃
14.7 g NaHCO₃
Step 2: Define conversions
Molar Mass of Na - 22.99 g/mol
Molar Mass of H - 1.01 g/mol
Molar Mass of C - 12.01 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of NaHCO₃ - [22.99 + 1.01 + 12.01 + 3(16.00)] g/mol = 84.01 g/mol
1 L = 1000 mL
Step 3: Find moles of solute
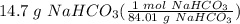 = 0.174979 mol NaHCO₃
= 0.174979 mol NaHCO₃
Step 4: Find amount of solution
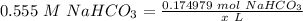
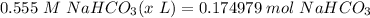
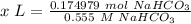
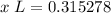
Step 5: Convert
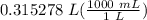 = 315.278 mL
= 315.278 mL
Step 6: Simplify
We are given 3 sig figs.
315.278 mL ≈ 315 mL