Answer:
0.8M
Step-by-step explanation:
Given parameters:
Number of moles of KNO₃ = 0.2mol
Volume of KNO₃ = 250mL
Unknown:
Molarity of the solution = ?
Solution:
The molarity of a solution is the number of moles in a given volume of a solution;
Molarity =
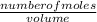
We need to convert the given volume to L;
1000mL = 1L
250mL will give 250 x 10⁻³L = 0.25L
So;
Molarity =
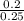 = 0.8M
= 0.8M