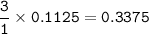Moles of Sr²⁺ = 0.3375
Further explanation
Molarity is a way to express the concentration of the solution
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
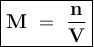
Where
M = Molarity
n = Number of moles of solute
V = Volume of solution
So to find the number of moles can be expressed as
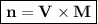
Strontium phosphide (Sr₃P₂)
Sr₃P₂ ⇒ 3Sr ²⁺ + 2P³⁻
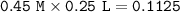
mol ratio Sr₃P₂ : Sr ²⁺ = 1 : 3