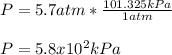Answer:
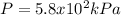
Step-by-step explanation:
Hello.
In this case, since chlorine gas is assumed to behave ideally, we can use the ideal gas equation in order to compute the required pressure:
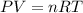
In such a way, since the temperature must be in kelvins and the volume in liters for the universal gas constant:
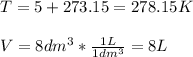
Thus, we first compute the pressure in atm:
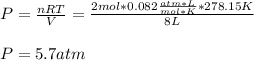
Thus, the pressure in kPa turns out: