Answer:
Q = -627.6 J
Step-by-step explanation:
Mass, m = 15 grams
Initial temperature,
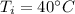
Final temperature,
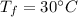
We need to find the amount of heat released by this much of sample of water. We know that heat released is given by the below formula :
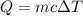
c is specific heat of water,
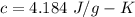
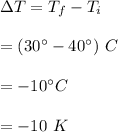
So,
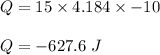
Hence, 627.6 J of heat is released and negative sign shows that energy is being given off.