Answer:
The answer is 85.71 mL
Step-by-step explanation:
The new volume can be found by using the formula for Boyle's law which is
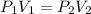
where
P1 is the initial pressure
P2 is the final pressure
V1 is the initial volume
V2 is the final volume
Since we are finding the new volume
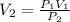
We have
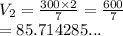
We have the final answer as
85.71 mL
Hope this helps you