The chemical equation is balanced
Further explanation
The chemical equation is said to be balanced if the number of atoms in the reactants and the number of atoms in the product is the same.
Balancing the equation can be done by adjusting the reaction coefficients in the products and reactants and not changing the chemical formula
This method can be done by balancing each atom first by giving a coefficient of 1 on the most complex compound
Reaction
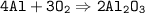
To check if this equation is balanced, take a look at the number of each of the atoms in the products and reactants
- Al : left=4, right=4
- O : left=6, right=6
Because the number of atoms is the same, it can be said that the chemical equation above is balanced