Answer:
The amount of energy absorbed when 10 g water at 86 C turns completely to steam vapor at 100 C is 584.76 J
The amount of energy released when 10 g water at 72 C freezes completely at 0 C is 3,012.48 J
Step-by-step explanation:
Sensible heat is the amount of heat that a body can receive without affecting its molecular structure. If the molecular structure does not change, the state (solid, liquid, gas) does not change. Since the molecular structure does not change, a change in temperature is observed.
In other words, the heat that produces a change in the temperature of a substance without any changes in its physical state (phase change) is called sensible heat.
The equation that allows calculating this type of heat exchange is:
Q = c * m * ΔT
where Q is the heat exchanged by a body of mass m, constituted by a substance of specific heat c and where ΔT is the change in temperature.
1. In this case, you know:
- c= 4.184
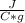
- m= 10 gr
- ΔT=Tfinal - Tinitial= 100 C- 86 C=14 C
Replacing:
Q= 4.184
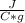 *10 g*14 C
*10 g*14 C
Solving:
Q= 584.76 J
The amount of energy absorbed when 10 g water at 86 C turns completely to steam vapor at 100 C is 584.76 J
2. In this case, you know:
- c= 4.184
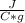
- m= 10 gr
- ΔT=Tfinal - Tinitial= 0 C- 72 C= -72 C
Replacing:
Q= 4.184
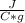 *10 g*(-72) C
*10 g*(-72) C
Solving:
Q= -3,012.48 J
The amount of energy released when 10 g water at 72 C freezes completely at 0 C is 3,012.48 J