Answer:
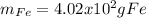
Step-by-step explanation:
Hello!
In this case, since the mole-mass relationships allows us to compute the moles or mass of a sample given the mass or moles via the atomic (elements) or molar mass (compounds). For iron, whose atomic mass is 55.847 g/mol, we can compute the mass o 7.20 moles as shown below:
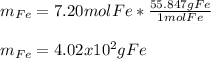
As you can see we write 1 mol Fe on bottom in order to cancel the given moles out.
Best regards!