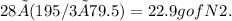Answer:
→ 22.9 g of N2.
Step-by-step explanation:
→ The molar mass of CuO is 63.5 + 16 = 79.5
→ The molar mass of N2 is 2×14 = 28
According to the equation, for every 3 molecules of CuO, one molecule of N2 is made.
Therefore,
3×79.5 g of CuO make 28 grams of N2.
Therefore,
195 g of CuO make