Answer:
The answer is 8.54 g/cm³
Step-by-step explanation:
The density of a substance can be found by using the formula
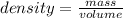
From the question
volume = final volume of water - initial volume of water
volume = 60.5 - 50 = 10.5 mL = 10.5 cm³
We have
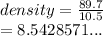
We have the final answer as
8.54 g/cm³
Hope this helps you