Answer:
You must add 53,295 J.
Step-by-step explanation:
Calorimetry is the measure of the amount of heat that a body gives up or absorbs in the course of a physical or chemical process.
When the heat added or removed from a substance causes a change in temperature in it, it is called sensible heat. In other words, sensible heat is the amount of heat absorbed or released by a substance when a change in temperature occurs, without a change in its state. Its mathematical expression is:
Q = c * m * ΔT
where Q is the heat exchanged by a body of mass m, constituted by a substance of specific heat c and where ΔT is the variation in temperature.
In this case:
- Q=?
- c= 4.18
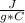
- m=150 g
- ΔT= Tfinal - Tinitial= 100 °C - 15 °C= 85 °C
Replacing:
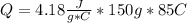
Solving:
Q= 53,295 J
You must add 53,295 J.