Answer:
4.81 g / mol
Step-by-step explanation:
Given :
Titanium has an HCP unit cell
Radius of titanium, R = 0.1445 nm
Unit cell volume,
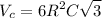
But for Ti, c/a = 1.58
So, c = 1.58 a
And a = 2R or c = 3.16 R
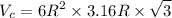
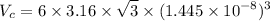
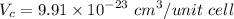
Density of Ti (theoretical),
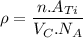
For HCP, n = 6 atoms per unit cell and atomic mass = 47.87 g/mol
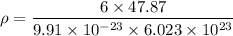
= 4.81 g/mol
This is the theoretical density of titanium.
The value given in literature is 4.51 g/mol