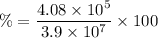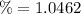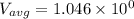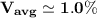Here is the full question:
An atom of argon has a radius of 71 .pm and the average orbital speed of the electrons in it is about 3.9 × 10⁷ m/s. Calculate the least possible uncertainty in a measurement of the speed of an electron in an atom of argon. Write your answer as a percentage of the average speed, and round it to significant digits.
Answer:
Step-by-step explanation:
From the above information:
The radius of an atom of argon = 71 .pm = 71 × 10⁻¹² m
The diameter of the atom of argon Δx =142 × 10⁻¹² m
According to Heisenberg's Uncertainty Principle,
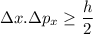
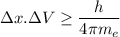
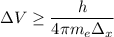
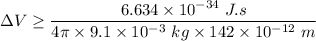
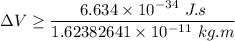
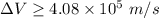
where; the average speed
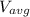 = 3.9 10⁷ m/s
= 3.9 10⁷ m/s
∴
The percentage of the average speed is expressed as a fraction of: