Answer:
Step-by-step explanation:
The Chromite ore is used for the production of Chromate/dichromate formation.
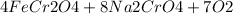 →
→
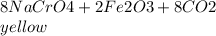
so
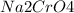 is called Chromite ore which is of yellow color.
is called Chromite ore which is of yellow color.
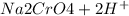 →
→
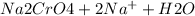
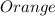
so when sodium chromate is reacted with hydrogen ion it forms sodium dichromate which is of orange color
Now
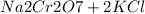 →
→
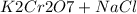
The chromate and dichromate are interconvertible in aqueous solution depending upon the PH of solution
Acid medium:
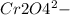
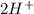 →
→
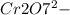
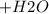
Base:
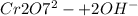 →
→
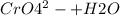
Hence Chromate and dichromate are interconvertible and hold and equilibrium.