Answer:
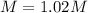
Step-by-step explanation:
Hello.
In this case, since the molarity of a solution is computed as the moles of solute over volume of solution:
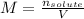
Since the volume of the solution is 257 mL or also 0.257 L, we compute the moles of solute, which is magnesium nitrate (molar mass = 148.3 g/mol), as shown below:
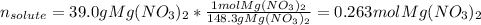
Thus, the required molarity turns out:
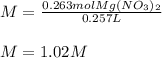
Best regards.