Answer:
0.060 g
Step-by-step explanation:
Hydrogen gas is made up of 2 atoms of hydrogen
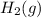 .
.
From the formula for calculating mole:
mole = mass/molar mass
Molar mass of hydrogen gas = 2.016 g/mol
Hence, 0.030 mole hydrogen gas would weigh:
mass = molar mass x mole
= 2.016 x 0.030
= 0.06048 g
The mass of hydrogen gas that participated to the correct number of significant digits is, therefore, 0.060 g