Answer:
See full explanation.
Step-by-step explanation:
Hello.
In this case, since we apply the law of mass action in order to write the equilibrium expression for each chemical reaction, we proceed as follows:
a. The reaction is:
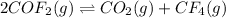
And the equilibrium expression:
![Kc=([CO_2][CF_4])/([COF_2]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/rrthqwx8wn9a3kam6c6s4qwora8mksr62s.png)
Since all the species are in gaseous phase they are included on the equilibrium expression.
b. The reaction is:
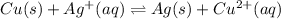
And the equilibrium expression:
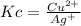
Since only copper (II) ions and silver (I) ions are in aqueous phase.
c. The reaction is redox and it turns out:
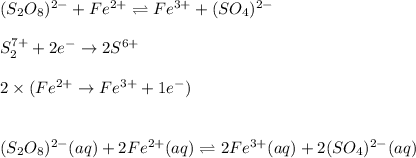
And the equilibrium expression:
![Kc=([Fe^(3+)]^2[(SO_4)^(2-)]^2)/([(S_2O_8)^(2-)][Fe^(2+)]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/7yiqis92g5n8ds89xughkn4jdgntkty16w.png)
Since all the species are in aqueous phase.