Answer:
The charges are typically used to determine the mole ratio and composition of the individual atoms of elements in the compound
Step-by-step explanation:
Take for instance the following compounds:
 and
and
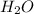
- Na has a charge of +1 and OH -1. equal charge means equal composition.
- H has charge of +1 while O has charge of -2. The charges are unequal, so the mole ratio for conversion would require multiplication by 2...which makes H two atoms but oxygen one atom in the compound.
I hope this helps.