Answer: The atomic mass of silver is 107.8
Step-by-step explanation:
Average atomic mass is the average mass of all the isotopes present depending on the relative abundance of each isotope.
Mass of isotope A = 106.9051
% abundance of isotope 1 = 51.82% =
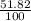
Mass of isotope B = 108.9047
% abundance of isotope 2 = (100-51.82)% =
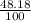
Formula used for average atomic mass of an element :
![A=\sum[(106.9051)* (51.82)/(100))+(108.9047)* (48.18)/(100)]]](https://img.qammunity.org/2021/formulas/chemistry/college/6tj5ph41j90wjokvashlj3j868cs0b11el.png)
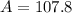
Therefore, the average atomic mass of silver is 107.8