Answer:
About 21.7 grams.
Step-by-step explanation:
To find the mass of a sample of sodium chlorate (NaClO₃) given the amount of particles present, we can convert from particles to moles, and moles to mass using its molecular weight.
The molecular weight of sodium chlorate is:
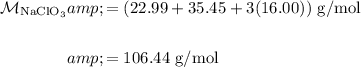
And recall that in one mole of any substance, there are 6.02 × 10²³ of it. Hence:
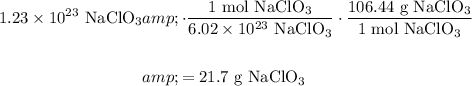
In conclusion, there are about 21.7 grams of sodium chlorate.