Answer:
B
Step-by-step explanation:
Recall the ideal gas law:
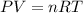
Because n, R, and T remain constant, we can rewrite the equation as:
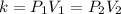
Where k is some constant. This form of the ideal gas law is referred to as Boyle's Law.
Substitute and evaluate:
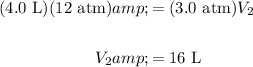
In conclusion, the answer is B .