Answer:
pH = 10.63.
Step-by-step explanation:
Recall that lithium hydroxide (LiOH) is a strong base and hence dissociates completely into Li⁺ and OH⁻ ions.
Therefore, the concentration of OH⁻ ions is equivalent to the concentration of the base.
Find pOH:
![\displaystyle \begin{aligned} \text{pOH} & = -\log[\text{OH}^-] \\ \\ & = -\log(0.00043) \\ \\ & = 3.37 \end{aligned}](https://img.qammunity.org/2023/formulas/chemistry/high-school/ymae3t7kri2ff3y7c85kx2deiufga0mxa9.png)
Using the relationships between pH and pOH, hence determine pH:
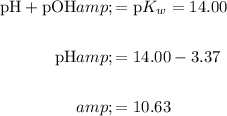
In conclusion, the pH of the solution is about 10.63.