Answer:
Electrolysis of Al₂O₃: 4Al³⁺ (s) + 6O²⁺ (g) → 4Al (s) + 3O₂ (g)
Reduction of Elemental Fe: 2Fe₂O₃ (s) + 3C (s) → 4Fe (s) + 3CO₂ (g)
Atom Economy for Electrolysis of Al₂O₃: 52.9227%
Atom Economy for Reduction of Fe₂O₃: 62.8534%
Step-by-step explanation:
Step 1: Define Compounds
Aluminum Oxide - Al₂O₃
Iron Oxide - Fe₂O₃
Step 2: RxN
Al₂O₃ (s) → Al (s) + O₂ (g)
Fe₂O₃ (s) + C (s) → Fe (s) + CO₂ (g)
Step 3: Balance RxN
2Al₂O₃ (s) → 4Al (s) + 3O₂ (g)
- We need the same number of O on both sides (6 is the LCM)
- We will also need to balance the number of Al on both sides due to the change of O (4 reactant/product)
This is ONLY the decomposition reaction for Aluminum oxide, NOT the electrolysis.
2Fe₂O₃ (s) + 3C (s) → 4Fe (s) + 3CO₂ (g)
- We need the same number of O on both sides (6 is the LCM)
- We will also need to balance the number of Fe on both sides due to the change of O (4 reactant/product)
- We will also need to balance the number of C on both sides due to the change of O (3 reactant/product)
This is the final single-replacement reaction for the reduction of Iron Oxide to Iron.
Step 4: Electrolysis of Al₂O₃
We will have to use oxidation-reduction reactions (half-reactions). Let's break up the reaction into it's elements.
Al³⁺ + ? → Al
- To make the ion Al³⁺ turn into its neutral atom, we will need to add 3e⁻ to balance the half reaction
Al³⁺ + 3e⁻ → Al
O²⁻ → O₂ + ?
- Oxygen is a diatomic element, and in it's natural state is bonded to itself. We need to balance the half reaction
2O²⁻ → O₂ + ?
- We need to figure out how much electrons the ion O²⁻ loses to turn into its neutral atom. We see that we will need to lose 4e⁻
2O²⁻ → O₂ + 4e⁻
Our half reactions:
Al³⁺ + 3e⁻ → Al
2O²⁻ → O₂ + 4e⁻
- We now need balance the entire half reaction. Our LCM is 12
4 (Al³⁺ + 3e⁻ → Al) = 4Al³⁺ + 12e⁻ → 4Al
3 (2O²⁻ → O₂ + 4e⁻) = 6O²⁻ → 3O₂ + 12e⁻
4Al³⁺ + 12e⁻ + 6O²⁺ → 4Al + 3O₂ + 12e⁻
- Cancel out spectator ions/e⁻ to get our final half reaction
4Al³⁺ (s) + 6O²⁺ (g) → 4Al (s) + 3O₂ (g)
Step 5: Atom Economy
According to GCSE and my own deciphering, your equation for Atom Economy is essentially calculating for something similar to percent yield (but not quite).
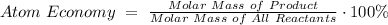
- 4Al³⁺ (s) + 6O²⁺ (g) → 4Al (s) + 3O₂ (g)
Molar Mass of Al - 26.98 g/mol
Molar Mass of O - 16.00 g/mol
Reactants: 4(26.98 g/mol) + 6(16.00 g/mol) = 203.92 g/mol
Products (Al as end product): 4(26.98 g/mol) = 107.92 g/mol
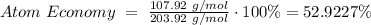
- 2Fe₂O₃ (s) + 3C (s) → 4Fe (s) + 3CO₂ (g)
Molar Mass of Fe - 55.85 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of C - 12.01 g/mol
Reactants: 4(55.85 g/mol) + 6(16.00 g/mol) + 3(12.01 g/mol) = 355.43 g/mol
Products (Fe as end product): 4(55.85 g/mol) = 223.4 g/mol
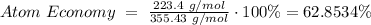
Step 6: Check for significant figures
Since we are not given any values, we don't really need to change any numbers to fit sig fig rules.