Answer: Molality of a saline solution = 1.14 moles per kg
Step-by-step explanation:
Given mass of NaCl (m)= 50.0 g
Mass of solvent = 0.750 kg
Molar mass of NaCl (M)= 23 g + 35.5 g = 58.5g [Atomic mass of Na = 23 u , atomic mass of Cl = 35.5 u]
Number of moles (n) =
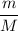
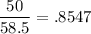
Molality =
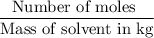
![=(0.8547)/(0.750)=1.1396\approx1.14\text{ moles per kg]]()
Hence, molality of a saline solution = 1.14 moles per kg